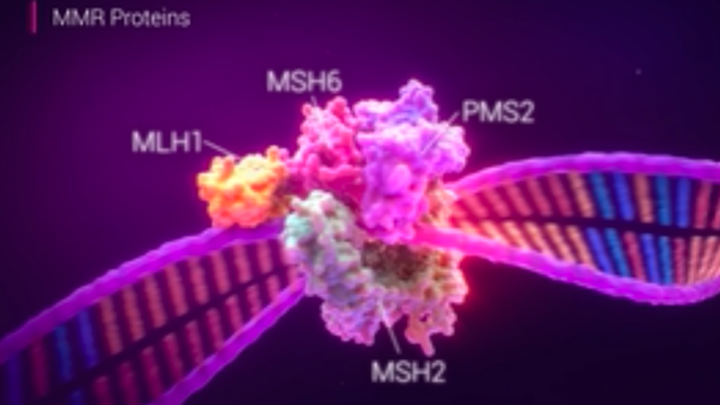
Новое исследование предполагает, что маркеры длинных мононуклеотидных повторов имеют преимущества в исследованиях микросателлитной нестабильности при множественных раковых заболеваниях
В новом исследовании, опубликованном в Journal of Molecular Diagnostics, подчеркивается потенциал использования маркеров длинных мононуклеотидных повторов (LMR) для характеристики микросателлитной…